Is there cure for cerebral palsy?
A study has been done on the efficacy of human umbilical cord blood mesenchymal stem cell infusion for children with cerebral palsy. CP currently has two generally successful methods of treatment, stem cell therapy and rehabilitation. Typically, in stem cell therapy it is more convenient to use human umbilical cord blood-derived MSCs (hUCB)-MSCs), as their use encounters fewer ethical dilemmas, the cells have comparatively low immunogenicity as well as immunosuppressive ability (meaning they are less likely to produce an immune response), and they have a higher rate of proliferation. Due to these comparative benefits and potential, clinical research has been performed to study the safety and efficacy of hUCB-MSC infusion in children with CP, and the results have shown promise in some cases. Furthermore, because both stem cell therapy and rehabilitation have shown positive results, combined treatment is a commonly preferred option, as significant improvement in motor function can be achieved when both therapies are combined. This combination method was chosen to be used for this study. Stem cell treatment for cerebral palsy
Clinical trial
The trial was designed to be a placebo-controlled, single-blind study. 56 children were enrolled in the recruitment phase starting September 2010, and lasted until the last follow-up visit in September 2015. Only 2 of the 56 dropped out before the second course. All patients were randomly assigned evenly between 2 groups, one that had hUCB-MSC injections with rehabilitation and one that had normal saline (0.9% NS) injections with rehabilitation. All patients and family were blinded to the group assignment, but investigators and charge nurses were made aware of the treatment information so that they would be prepared to handle any emergencies if needed.
The allogeneic hUCB-MSCs used in the study were acquired from the UCB bank of Beike Biotechnology Company (Shenzhen, China). All manufacturing processes and laboratories met the standards for good manufacturing practices and good tissue practices. The resources were from the umbilical cord blood and tissues of healthy puerperal women. The women were checked and confirmed negative for syphilis, HIV, hepatitis B virus (HBV), toxoplasma, nubellavirus, cytomegalo virus, herpes simplex virus, and other viruses.
Stem cell research for cerebral palsy cure
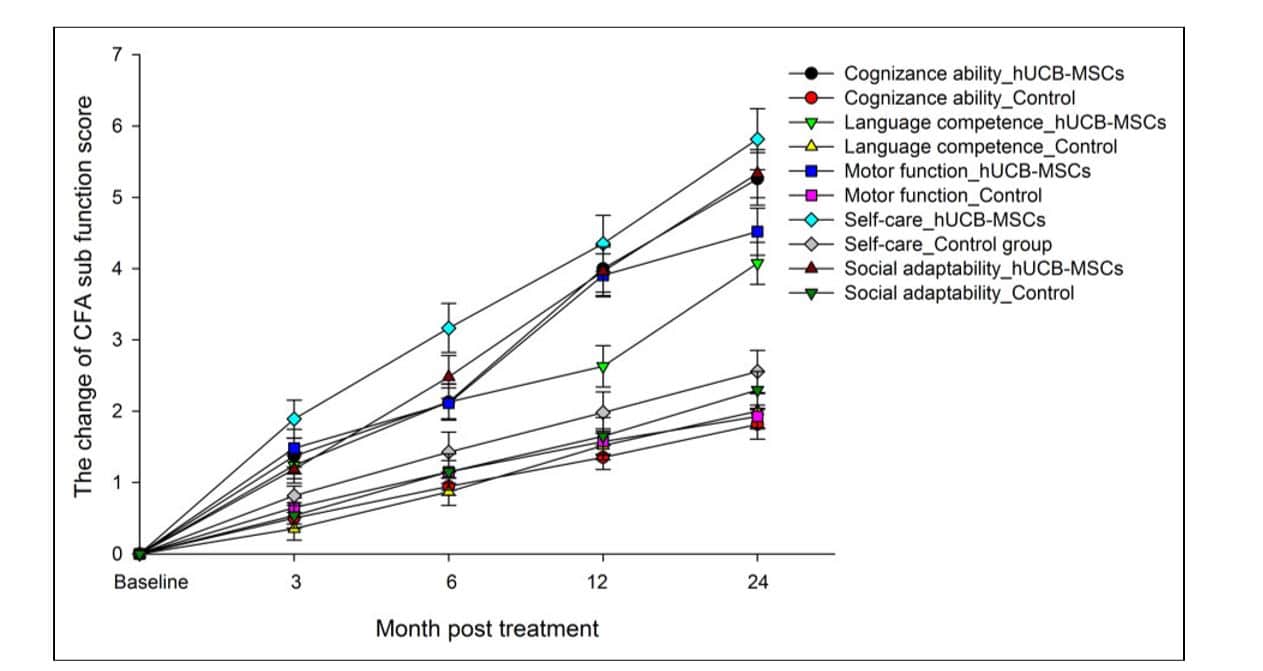
The initial evaluation before treatment between the two groups showed very little difference in both the Gross Motor Function Measure (GMFM-88) scores and the comprehensive functional assessment (CFA) scores. However, as the treatment and trial progressed the efficacy became much more significant for the infusion group as opposed to the placebo group, as observed at the 3rd, 6th, 12th, and 24th month of post treatment follow up. The GFMF-88 score, which assesses 5 function areas, (“lying and rolling,” “sitting,” “crawling and kneeling,” “standing,” and “walking, running, and jumping”), showed significant efficacy in the infusion group, but failed to show any substantial improvement in the control group. The CFA scale, which assesses cognizance, language competence, self-care, motor function, and social adaptability, showed its effective status 6 months earlier than the GMFM-88 scale. This shows that patients could experience more comprehensive improvements before they began to show improvements in gross motor function.
A graph of the changes in the GMFM-88 scores can be seen in Figure 2 below:

Further data showing the improvements in CFA scores can be seen below in Figure 4:
Please click the link below for more in-depth information on the study.
